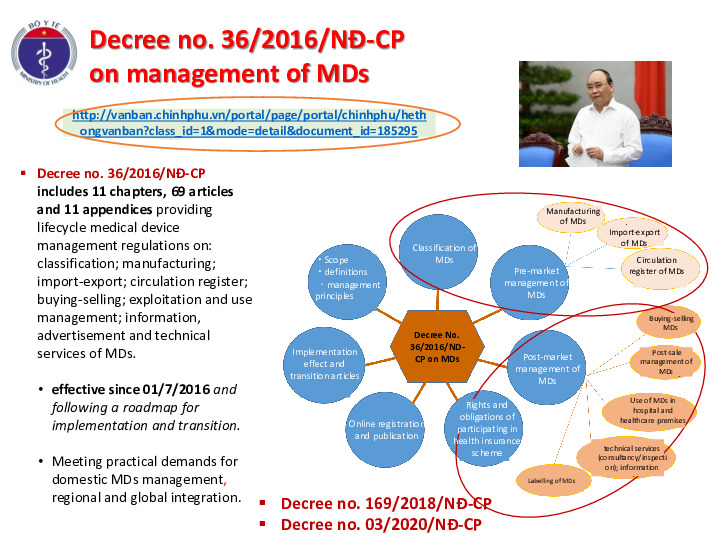
Decree no. 36/2016/NĐ-CP includes 11 chapters, 69 articles and 11 appendicesproviding lifecycle medical device management regulations on: classification; manufacturing; import-export; circulation register; buying-selling; exploitation and use management; information, advertisement and technical services of MDs
よくある質問をご覧いただき、ご質問が解決しない場合は、下記お問い合わせフォームよりお問い合わせ下さい。

