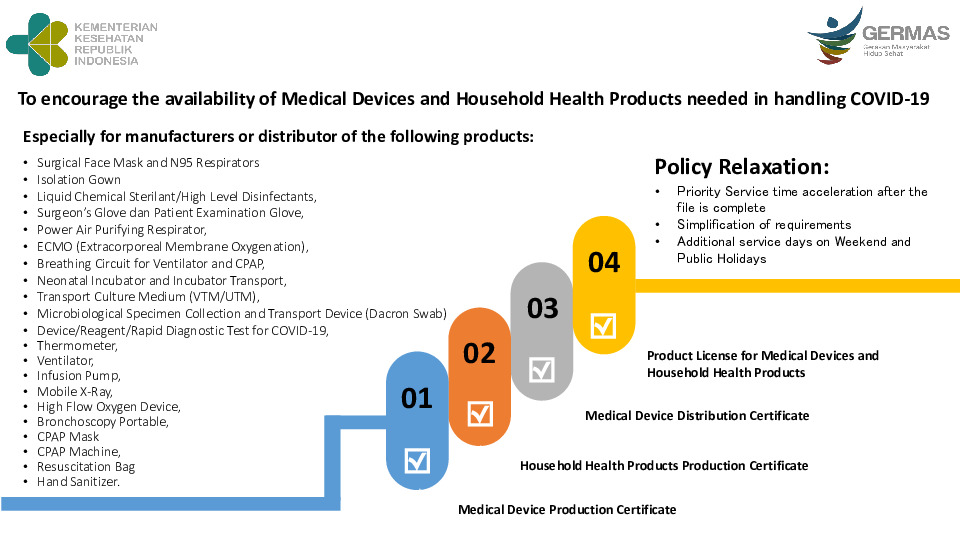
Especially for manufacturers or distributor of the following products: Product License for Medical Devices and Household Health Products To encourage the availability of Medical Devices and Household Health Products needed in handling COVID-19 •Surgical Face Mask and N95 Respirators •Isolation Gown •Liquid Chemical Sterilant/High Level Disinfectants, •Surgeon’s Glove dan Patient Examination Glove, •Power Air Purifying Respirator, •ECMO (Extracorporeal Membrane Oxygenation), •Breathing Circuit for Ventilator and CPAP, •Neonatal Incubator and Incubator Transport, •Transport Culture Medium (VTM/UTM), •Microbiological Specimen Collection and Transport Device (Dacron Swab) •Device/Reagent/Rapid Diagnostic Test for COVID-19, •Thermometer, •Ventilator, •Infusion Pump, •Mobile X-Ray, •High Flow Oxygen Device, •Bronchoscopy Portable, •CPAP Mask •CPAP Machine, •Resuscitation Bag •Hand Sanitizer.
よくある質問をご覧いただき、ご質問が解決しない場合は、下記お問い合わせフォームよりお問い合わせ下さい。

